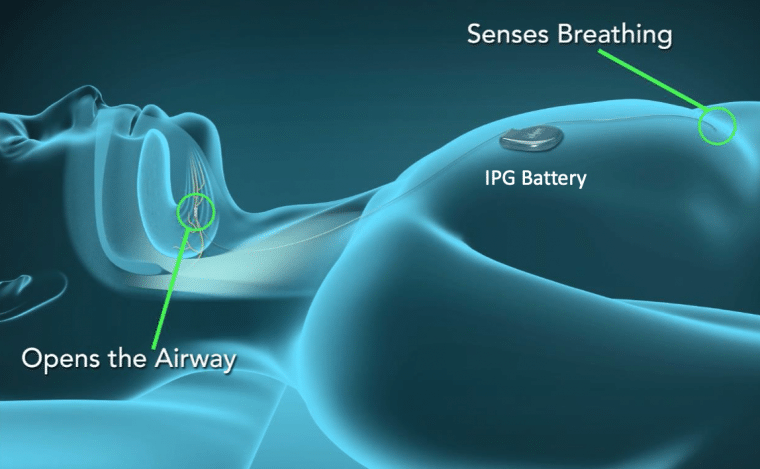
This sounds like a novel idea, but it was first described many years ago by Johns Hopkins researchers in the 1990s. It didn’t take hold for mass sleep apnea treatment until 3 start-up companies were created in the United States in 2006 years of 2007. ImThera is being used in Europe and is currently in clinical trials in the United States. Inspire and Apnex performed clinical studies in the early 2010s.
Inspire received FDA approval in 2014. In their landmark New England Journal of Medicine publication, they found that in the 126 patient study, the average AHI at 12 months dropped from 29.3 to 9.0, a 68% difference. The overall response rate (> 50% drop in AHI and final AHI < 20) was 66%. The Epworth Sleepiness Score dropped from 11.6 to 7 (< 10 is considered normal). Functional Outcome of Sleep Questionnaire (FOSQ) scores increased from 14.3 to 17.3 (>2 point improvement is considered clinically significant). Currently, Inspire is the only hypoglossal nerve stimulation technology that is approved for patient use in the United States.
Being a bit skeptical about new devices coming out in our field, I waited to see how Inspire performed, especially with long term outcomes, as well as complication rates. I was pleased to see that they published numerous long-term follow-up studies, even up to the 5 year mark. For the most past, all their studies showed improvements seen initially persist up to the 5 year mark. A systematic review and meta-analysis of all available studies in 2019 found that overall, the AHI dropped 21 points, Epworth Sleepiness Score dropped 5 points, and the FOSQ increased 3 points. More and more insurances are now beginning to cover this new technology. Well over 90% of patients are satisfied with the procedure, snoring elimination, and prefer it to CPAP.
Based on all these positive results, here’s the bad news: You have to meet very specific criteria to be eligible for this treatment. Specifically, these are the requirements:
- Refuse or can’t tolerate CPAP
- BMI < 32
- Age > 22
- AHI between 15 and 65
- And sleep endoscopy showing front to back palate collapse only.
You can also imagine that some people just won’t like the idea of an implant of any kind in the chest wall, with small incisions in the neck and chest walls. Unfortunately, many people won’t meet the weight cutoff of 32. Even if you meet all the other criteria, you still need to undergo drug-induced sleep endoscopy to confirm if you are a candidate. This is a short procedure that’s performed in the operating room using an IV infusion of a sleep-inducing medication, with a flexible fiberoptic camera placed through the nose to look at your throat while you’re sleeping. To be a candidate, your soft palate must collapse in a front-to-back manner, and not in a pursestrings-like manner. In my experience, not too many people will qualify based on this particular test. Lastly, you could be in the 10-20% where you don’t get the result that you want, or suffer from serious complications (which are low at 2%). These include device failure, infections or nerve injury.
The procedure is performed as an outpatient procedure, taking about 2 hours to perform. The battery lasts about 10 years. You will be given a remote control to turn it on or off, as well as to control various settings. One post-op visit is needed to see your surgeon. Four weeks later, you will see your sleep doctor in a designated Inspire trained sleep lab to turn on the device, after which a final overnight sleep study is performed to fin-tune the device, like a CPAP titration. Contrary to what you may think, the nerve stimulation level is similar to what your brain sends to your tongue, so you won’t feel any “shocks.” F
If you don’t meet the criteria for this procedure, there are various other ways of treating this condition, including other surgical procedures, dental appliances, and ways of getting you to improve your CPAP experience. Whatever you do, don’t give up, especially if you have moderate to severe obstructive sleep apnea. I generally recommend trying non-surgical options first, especially dental options. However, some people can’t tolerate CPAP or don’t have success with dental appliances. This new procedure is definitely not hype, but can be a hopeful option in some selective patients.
** This post was originally published on http://feedproxy.google.com/~r/doctorpark/~3/x4k-ev-fjiU/inspire